ALG.APV-527
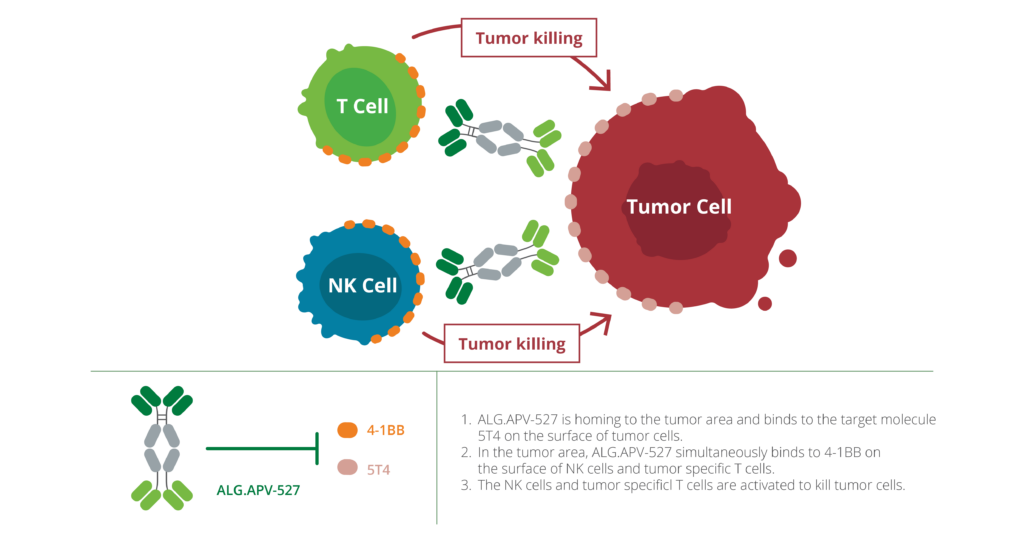
ALG.APV‑527 is a bispecific antibody co‑targeting the immune costimulatory receptor 4‑1BB and the tumor antigen 5T4. Designed to selectively activate T cells and NK cells within 5T4-expressing tumors, ALG.APV‑527 leverages tumor-directed activation to minimize off‑tumor effects and enhance anti‑tumor responses in hard‑to‑treat solid cancers.
ALG.APV-527: Tumor-directed immunotherapy targeting 4-1BB and 5T4
ALG.APV-527 is a bispecific antibody designed to activate T cells and NK cells, stimulating tumor-specific immune responses. It binds simultaneously to 4-1BB, a co-stimulatory receptor, and 5T4, a tumor-associated antigen expressed on several cancers, including triple-negative breast cancer and renal cell carcinoma. This dual binding ensures immune activation occurs only in the tumor microenvironment, which helps maintain a favorable balance between efficacy and safety.
In July 2017, Aptevo Therapeutics Inc. and Alligator signed an agreement regarding the co-development of ALG.APV-527. Under the agreement, both companies will own and finance the development equally (50/50). The original molecules of the tumor-binding and immunomodulatory parts of ALG.APV-527 was developed using Alligator’s proprietary ALLIGATOR-GOLD® antibody library. Aptevo then enhanced the bispecific format using its ADAPTIR™ platform. By combining these technologies, the companies created a drug candidate that selectively targets tumors and stimulates local anti-tumor immune cells.
Clinical development and preclinical insights
Preclinical data, presented at multiple international oncology conferences, show that ALG.APV-527 selectively enhances T cell responses within the tumor. In November 2022, consolidated data was published in Molecular Cancer Therapeutics1, confirming that ALG.APV-527 can eliminate tumors and induce long-lasting immunologic memory in disease models. Notably, the safety profile was strong, with no signs of systemic immune activation or liver toxicity.
Phase 1 clinical progress
In February 2023, the first patient was dosed in a Phase 1 trial evaluating safety and early efficacy in up to 30 patients with solid tumors that overexpress 5T4. The trial enrolled heavily pretreated patients, including those with breast cancer. By March 2024, interim data showed encouraging safety and pharmacokinetics, along with early signals of clinical activity. In Q4 2024, the companies reported that ALG.APV-527 met its Phase 1 objectives for exposure, tolerability, biological activity, and overall safety.
1 Mol Cancer Ther. 2023 Jan 3;22(1):89-101. DOI: 10.1158/1535-7163.MCT-22-0395.