ALG.APV-527
Result of a strong collaboration
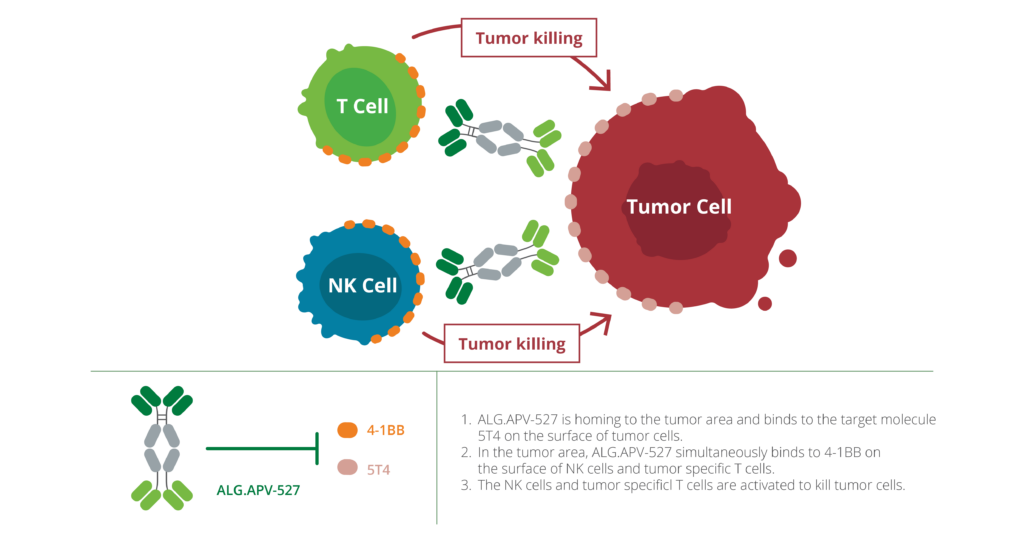
ALG.APV-527 is a bispecific antibody that targets the 4-1BB and 5T4 molecules and is expected to stimulate T cells and NK cells driving tumor specific immune attacks as described for ATOR-1017 above. 5T4 is a protein preferentially expressed on several tumor types including triple negative breast cancer and renal cell carcinoma. ALG.APV-527 requires simultaneous binding to 4-1BB and 5T4 to stimulate T cells and NK cells, thereby securing that it will only drive immune responses in the tumor and not elsewhere in the body, thus securing a favorable balance between efficacy and safety.
ALG.APV-527 is designed for the treatment of metastatic cancer and has been co-developed with Aptevo Therapeutics Inc. since 2017. During Q3 2022, the IND for ALG.APV-527 was cleared by the FDA, and the first patient in the Phase 1 study conducted in the US was dosed during February 2023.
Project status
Project status: Phase 1 study initiated
In recent years, preclinical data for ALG.APV-527 has been presented at several international conferences. In November 2022, consolidated preclinical data was published in the peer-reviewed journal Molecular Cancer Therapeutics.1 The data demonstrates that ALG.APV-527 effectively and selectively stimulates and strengthens the T cell response in the tumor, leading to tumor elimination. ALG.APV-527 also induces a tumor-specific immunologic memory in experimental disease models. Furthermore, the data shows that ALG.APV-527 has a good preclinical safety profile, with no signs of systemic immunostimulation or liver toxicity. Overall, the results support the potential of ALG.APV-527 to induce effective tumor-targeted immunostimulation with fewer adverse events.
During Q3 2022, Aptevo Therapeutics Inc. and Alligator submitted an IND application to the US FDA. Later in Q3 2022, the companies received a “may proceed notice” from the FDA, allowing the initiation of Phase 1 clinical studies in the US. The Phase 1 study will assess the safety and efficacy of ALG.APV-527 in up to 30 patients with solid tumor types over-expressing 5T4. The first patient in the study was dosed with ALG.APV-527 in February 2023. In March 2024, the Company announced the first interim data from the Phase 1 study with more than half of the planned patients recruited. The data demonstrated an encouraging safety and pharmacokinetics profile for ALG.APV-527, as well early signs of clinical efficacy in heavily pretreated breast cancer patients.
Alligator expects to announce topline data from the study during H2, 2024.
Co-development with Aptevo Therapeutics Inc.
In July 2017, Aptevo Therapeutics Inc. and Alligator signed an agreement regarding the co-development of ALG.APV-527. Under the agreement, both companies will own and finance the development equally. The original molecules involved in the tumor-binding function and the immunomodulatory function of ALG.APV-527 were developed using Alligator’s patented ALLIGATOR-GOLD® antibody library. The bispecific molecule was further developed and improved with Aptevo’s technology platform ADAPTIR®. A tumor-binding function was combined with an immunomodulatory function in the same molecule to create a drug candidate that can selectively target the tumor and stimulate the antitumor-specific immune cells that are found there.
1 Nelson, M.H., Mol Cancer Ther. 2023, 22(1):89-101; https://doi.org/10.1158/1535-7163.mct-22-0395