Mitazalimab
Mitazalimab is Alligator’s lead CD40‑agonist, currently Phase 3‑ready in metastatic pancreatic cancer. Clinical results from the OPTIMIZE‑1 Phase 2 study demonstrate a threefold improvement in 24‑month survival versus standard therapy, supporting its advancement into pivotal development. Mitazalimab represents a high‑impact candidate within a tumor‑directed immunotherapy portfolio.
Project snapshot
Target: CD40
Indication: metastatic pancreatic cancer (initially)
Phase: Phase 3-ready
Key data: 24‑month survival rate 29.4% vs ~8%; ORR 42.1% confirmed
Regulatory status: FDA/PEI Phase 3 supportive feedback received early 2025
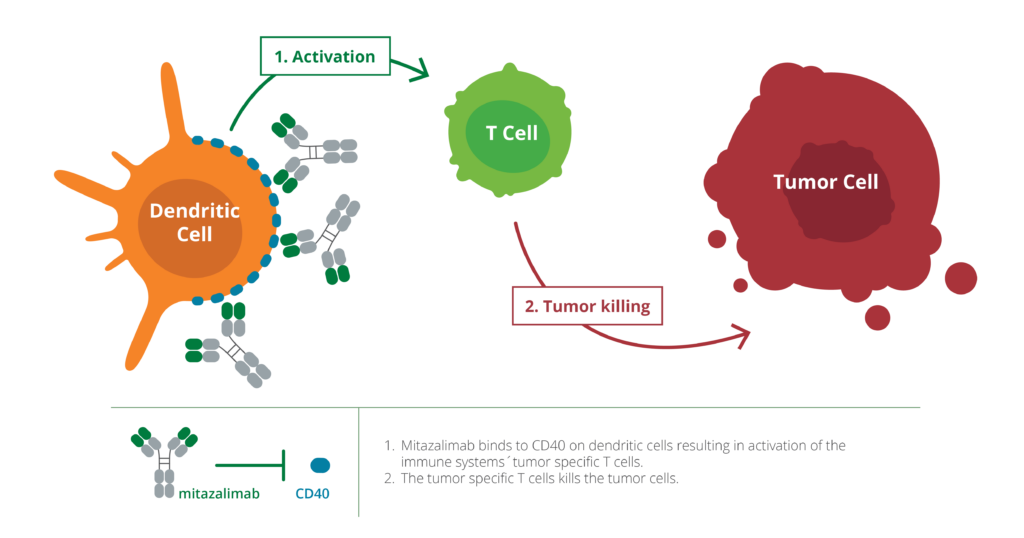
Mitazalimab is a stimulatory antibody targeting CD40, a receptor on dendritic cells of the immune system, which play a crucial role in recognizing cancer cells in the body. By activating CD40, mitazalimab enhances the ability of the dendritic cells to stimulate the immune system’s key weapons—T cells—allowing for a more effective and tumor-specific immune attack. In preclinical models, mitazalimab has been shown to induce a potent tumor-directed immune response and provide long-lasting tumor immunity. Furthermore, preclinical results indicate that mitazalimab has the potential to be used across multiple cancer types, and in combination with a variety of other treatments including chemotherapy, vaccines and check-point inhibitors.
Phase 2 in pancreatic cancer
To date, two clinical Phase 1 studies and one clinical Phase 2 study have been conducted with mitazalimab. The first Phase 1 study, conducted by Alligator, focused on intratumoral
administration. The second Phase 1 study, conducted by Janssen Biotech, Inc. in patients with various solid tumors, demonstrated that mitazalimab is safe and well tolerated at clinically relevant dose levels. Additionally, early signs of clinical activity were observed, including a partial response in a renal cancer patient and stable disease for at least six months in ten patients.1 Mitazalimab has also been evaluated in combination with the cancer vaccine MesoPher in an investigator-initiated Phase 1 study, REACTIVE-2, in patients with previously treated metastatic pancreatic cancer, where the last patient was dosed in 2023.
Biomarker data from the Phase 1 study confirmed mitazalimab’s mechanism of action, showing activation of macrophages, dendritic cells, and T cells—key components in the destruction of tumor cells and the achievement of clinical responses.1 These findings were further validated in a study analyzing gene transcription in immune cells from patients following mitazalimab administration.2 Collectively, these bio-marker data provide strong validation of mitazalimab’s ability to activate the immune system in cancer patients.
The Phase 2 clinical study OPTIMIZE-1 is an open-label, multi-center study evaluating the safety and efficacy of mitazalimab in combination with the chemotherapy regimen mFOLFIRINOX in previously untreated patients with metastatic pancreatic cancer. Clinical data from the study have demonstrated that mitazalimab, when combined with mFOLFIRINOX, provides significant survival benefits compared to standard of care.
Project status
Recently reported results
- The survival rate at 24 months was 29.4 percent in patients treated with mitazalimab in combination with mFOLFIRINOX, a threefold increase compared to the estimated 8 percent for chemotherapy FOLFIRINOX alone3.
- Median Overall Survival (mOS) was 14.9 months4, a strong outcome compared to 11.1 months reported for FOLFIRINOX4 and more recently for NALIRIFOX5.
- At the analysis cutoff at 24 months, 16 patients (28 percent) were still alive, and 5 (9 percent) remained on treatment. The longest ongoing treatment duration was 32 months.
- The confirmed Objective Response Rate (ORR) was 42.1 percent4, aligning well with the reported ORR of 31.6 percent in a similar patient population treated with FOLFIRINOX alone3, and the 42 percent ORR reported for NALIRIFOX5. The unconfirmed ORR was 54.4 percent among the 57 patients evaluated4.
- Median Duration of Response (DoR) was 12.6 months4, which was confirmed at the 24-month analysis — an exceptional result in this aggressive disease, significantly longer than the 5.9 months reported for FOLFIRINOX3 and 7.3 months reported for NALIRIFOX5.
Mitazalimab delivers long-term survival benefits when combined with chemotherapy
OPTIMIZE-1 is the first Phase 2 study with mitazalimab. The open-label, multi-center study evaluates the efficacy and safety of mitazalimab in combination with chemotherapy (mFOLFIRINOX) in patients with metastatic pancreatic cancer who have not previously received chemotherapy. We have continuously reported promising data from the study, which has evaluated 57 patients. The recently reported positive 24-month follow-up readout in Q1 2025 marks a significant milestone that differentiates mitazalimab from many other experimental treatments developed for this challenging disease.
In response to recommendations from the U.S. FDA to ensure that mitazalimab is well-prepared for Phase 3 evaluation, Alligator has recruited patients for an additional 450 µg/kg dose cohort to support the candidate’s dose characterization. Top-line data were reported in February 2025, indicating a positive dose-response relationship for mitazalimab, further supporting the selection of 900 µg/kg as the Phase 3 dose.
Development beyond Phase 2
Through interactions with the FDA and European regulatory authorities during 2024 and Q1 2025, Alligator has established a clear approval pathway for mitazalimab in first-line metastatic pancreatic cancer, confirming that OPTIMIZE-1 is a Phase 3-enabling study, as announced in February 2025. A Chemistry, Manufacturing, and Controls (CMC) interaction with the FDA in December 2024 confirmed previous feedback from the German Paul Ehrlich Institute (PEI) and ensured that completed and planned CMC work also enable Phase 3 development. Following this positive feedback, Alligator has initiated the manufacturing of GMP material for the Phase 3 trial.
Alligator is planning to proceed directly to a global Phase 3 study with the potential for accelerated approval and is preparing for a partnership to initiate the study in the second half of 2025. To facilitate this, the OPTIMIZE-1 study was expanded in 2024 to include an additional 15 patients at the 450 µg/kg dose level, in alignment with FDA guidance from December 2023. Patient recruitment for this cohort was completed in July 2024, and data on treatment exposure and response were reported in February 2025. The results showed an objective response rate (ORR) of 22.7% (unconfirmed), compared to 54.4% for the 900 µg/kg dose, indicating a positive dose-response relationship for mitazalimab and further supporting the selection of 900 µg/kg as the Phase 3 dose.
The final Phase 3 study design was presented to the FDA at an “End of Phase 2 meeting” in January 2025. Both the FDA and PEI have now confirmed that the proposed Phase 3 design can serve as the basis for Biologics License Application (BLA) and Market Authorization Application (MAA) submissions.
1 Invest New Drug. 2023 Feb;41(1):93-104. doi: 10.1007/s10637-022-01319-2.
2 Cells. 2023 Sep 27;12(19):2365. doi: 10.3390/cells12192365.
3 N Engl J Med 2011; 364:1817-1825; doi: 10.1056/NEJMoa1011923.
4 Lancet Oncol. 2024 Jul;25(7):853-864; doi: 10.1016/S1470-2045(24)00263-8.
5 Lancet. 2023 Oct 7;402(10409):1272-1281; doi: 10.1016/S0140-6736(23)01366-1