ATOR-1017
ATOR-1017 is a monoclonal antibody that stimulates the 4-1BB receptor on T cells and NK cells in the tumor. The molecule is being developed for the treatment of metastatic cancer.
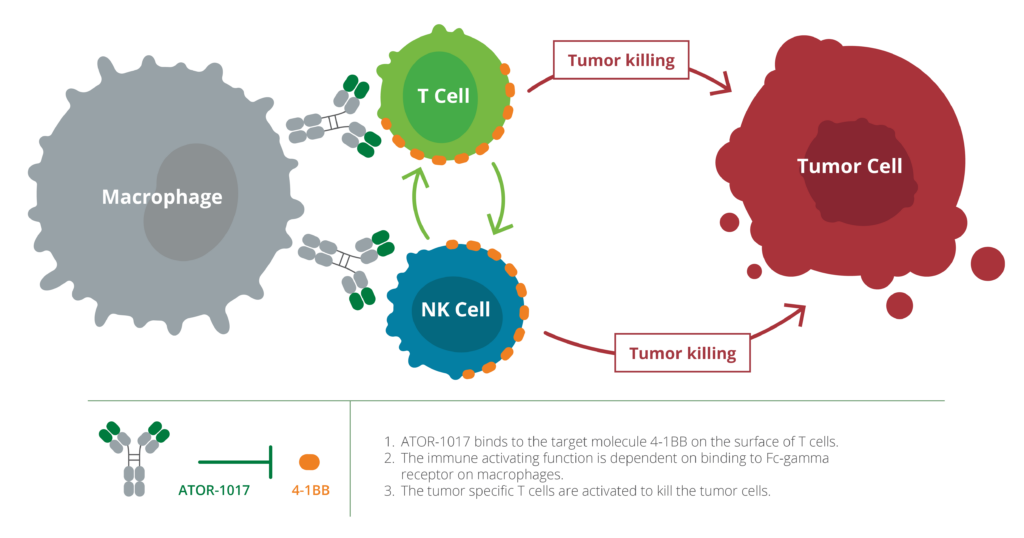
ATOR-1017 activates 4-1BB receptors, which increases the immune system’s ability to discover and kill tumor cells, making 4-1BB a highly interesting target for cancer immunotherapy. ATOR-1017 boosts immune responses in environments with high levels of immune cells, which occurs specifically in tumors. This creates an opportunity for potent, tumor-directed immunostimulation that can increase the effect and reduce side effects for the patient.
Project status
Encouraging clinical Phase 1 data
ATOR-1017 differs from other 4-1BB antibodies, partly because of its unique binding profile, and partly because its immunostimulatory function depends on crosslinking to Fc-gamma receptors on immune cells. This localizes the immunostimulation to the tumor region where both 4-1BB and Fc-gamma receptors are expressed at high levels.
Preclinical data have shown that ATOR-1017 stimulates both NK cells and T cells, both of which contribute to an effective immune-mediated killing of tumor cells. Stimulatory antibodies targeting 4-1BB therefore strengthen the ability of both NK cells and T cells to attack tumor cells. A Phase 1 dose-ranging study in patients with metastatic cancer was concluded during Q4 2022 with promising safety and pharmacology data, and Alligator is now in the process of identifying a partner before initiating Phase 2 clinical trials with ATOR-1017.
Clinical Phase 1 study completed
Since 2020, Alligator has provided regular updates on the safety and biomarker data from the ATOR-1017 Phase 1 study in patients with metastatic cancer.
In November 2022, the Company announced that the trial was completed and presented topline data from the study at the SITC meeting in Boston, United States. Data confirmed the favorable safety profile of the drug candidate with no severe immune-related adverse events reported even at the 900 mg top dose. Furthermore, the data validated ATOR-1017’s mechanism of action and showed that the drug candidate is pharmacologically active at doses above 100 mg. The study showed signs of clinical benefit, with ATOR-1017 providing a disease control rate of above 50 per cent, with six patients showing stable disease for more than six months. Two patients showed stable disease for more than 12 months, and two patients were still on study by 31 August 2022, the latest data cut-off date.
Alligator maintains a strong belief in the 4-1BB agonist field and ATOR-1017 and is looking for a partner for the project before initiating Phase 2 clinical trials with the molecule.