Mitazalimab
Mitazalimab is Alligator’s most advanced drug candidate designed for the treatment of metastatic cancers including pancreatic cancer.
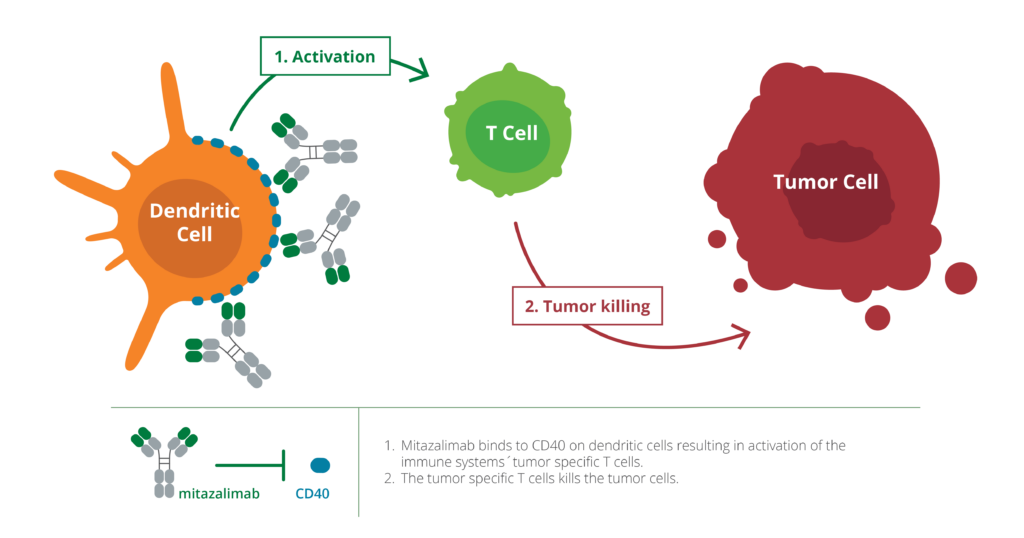
Mitazalimab is a stimulatory antibody that targets CD40, a receptor on the immune system’s dendritic cells, which are cells that recognize cancer cells in the body. Mitazalimab’s stimulation of CD40 enables the dendritic cells to activate the immune system’s weapons more effectively – in this case T cells – and to direct the immune system’s attack specifically to the cancer cells. Mitazalimab has been optimized using Alligator’s unique FIND® technology. In preclinical models, mitazalimab has been shown to induce a potent tumor-targeted immune response and provide long-lasting tumor immunity. Preclinical results have also shown that mitazalimab can be used to treat many different types of cancer.
Phase 2 in pancreatic cancer
To date, two clinical Phase 1 studies and one clinical Phase 2 study have been conducted with mitazalimab. The first study was conducted by Alligator with a focus on intra-tumoral administration. Clinical data from the second Phase 1 study conducted by Janssen Biotech, Inc. in patients with various solid tumors showed that mitazalimab is safe and well tolerated at clinically relevant dose levels. Early signs of clinical activity were also observed in the study – one renal cancer patient showed partial response, while ten patients maintained stable in their disease progression for at least six months.1
Biomarker data from the Phase 1 study confirmed mitazalimab’s mechanism of action, showing activation of macrophages, dendritic cells and T cells which is crucial for the destruction of tumor cells and eventually clinical response.1 These data were corroborated and extended in a study describing the pharmacodynamic changes by analyzing gene transcription in immune cells from patients after mitazalimab administration.2 Together, the biomarker data validates mitazalimab’s mechanism of action; activation of the immune systems in cancer patients.
The Phase 2 OPTIMIZE-1 clinical study was an open-label, multi-center study that assessed the safety and efficacy of mitazalimab (CD40 agonist) in combination with standard of care chemotherapy mFOLFIRINOX, in previously untreated, chemotherapy naive patients. Clinical data from the Phase 2 study demonstrated that mitazalimab in combination with mFOLFIRINOX provides significant survival benefit to pancreatic cancer patients compared to the standard of care.
Project status
Top-line data from clinical Phase 2 study received
OPTIMIZE-1 is the first Phase 2 study with mitazalimab. The study evaluates the efficacy and safety of mitazalimab in combination with chemotherapy (mFOLFIRINOX) in patients with metastatic pancreatic cancer. The first interim data from the study, published during Q3 2022, reconfirmed that mitazalimab is pharmacologically active and well tolerated also in combination with chemotherapy at 900 µg/kg, the highest dose tested. In January 2023, Alligator announced interim data from the first 23 evaluable patients treated with 900 µg/kg for 17 weeks. These data demonstrated that approximately 52 per cent of the patients responded to mitazalimab in combination with chemotherapy, as compared to the ~31 per cent response rate reported for FOLFIRINOX alone.3 Furthermore, 90 per cent of the patients showed clinical benefit from the combination at the 17-week timepoint. During April 2023, the Company announced that all patients have been recruited to OPTIMIZE-1 and reconfirmed the timelines towards interim data and topline readout, thus significantly reducing the operational risk in the clinical program. In June 2023, the Company extended the interim analysis to include the entire study cohort, showing an ORR of 44 per cent, and an encouraging durability of response of 8.7 months, compared to less than the six months reported for chemotherapy alone.3
Mitazalimab was granted orphan drug designation for treatment of pancreatic cancer on 18 May 2023 in the US and on 21 August 2023 in the EU.
In December 2023, Alligator discussed the continued development of mitazalimab in mPDAC with the US FDA. This dialogue confirmed that OPTIMIZE-1, if expanded with an additional of 15 patients at the 450 µg/kg dose, is Phase 3 enabling. Moreover, the dialogue confirmed a clear path to registration in pancreatic cancer based on a single randomized Phase 3 study, including the opportunity for accelerated approval.
On 29 January 2024, the Company released positive top-line results from the OPTIMIZE-1 Phase 2 study of the Company’s lead asset mitazalimab in first line metastatic pancreatic cancer. The open-label, multi-center study assessed the safety and efficacy of mitazalimab (CD40 agonist) in combination with standard of care chemotherapy mFOLFIRINOX, in previously untreated, chemotherapy naive patients.
The Phase 2 study achieved its primary endpoint with the top-line results demonstrating a confirmed ORR of 40.4 per cent, an unconfirmed ORR of 50.9 per cent and a DCR of 79 per cent in 57 evaluable patients, as per the Response Evaluation Criteria in Solid Tumors (RECIST 1.1). This compares favorably to the ORR of 31.6 per cent reported in a similar patient population treated with FOLFIRINOX alone.3
The cut-off time for analysis was 14 November 2023, with a median follow-up duration of 12.7 months. At the time of the analysis, a total of 29 (51 per cent) patients were still alive, of these 18 (32 per cent) were still on treatment. The longest ongoing treatment duration was 23 months. Three patients demonstrated complete remission of their target lesions. The study further demonstrated:
- Median Overall Survival (mOS) of 14.3 months at the time of analysis and is expected to improve as a majority of the patients remain alive, comparing favorably to the 11.1 months demonstrated by FOLFIRINOX3, and more recently by NALIRIFOX in the NAPOLI 3 Phase 3 trial.4
- An unprecedented median Duration of Response (DoR) of 12.5 months, compared to 5.9 months with FOLFIRINOX3, and the 7.3 months demonstrated by NALIRIFOX.4
- The 12-month survival rate was 59.3 per cent compared to 48.1 per cent for FOLFIRINOX3 and 45.6 per cent for NALIRIFOX.4
- Median Progression Free Survival (PFS) of 7.7 months, compared to 6.4 months with FOLFIRINOX3, and the 7.4 months demonstrated by NALIRIFOX.4
- Mitazalimab’s manageable safety and tolerability profile supporting long-term administration in combination with mFOLFIRINOX was confirmed.
In addition, mitazalimab is being tested in REACTIVE-2, an investigator-initiated Phase 1 trial led by investigators at Erasmus University Rotterdam, the Netherlands. REACTIVE-2 assesses the safety and efficacy of mitazalimab in combination with MesoPher, an experimental dendritic cell vaccine, in patients with pancreatic cancer. REACTIVE-2 will enroll up to 18 patients. REACTIVE-2 was fully enrolled in April 2023.
Development beyond Phase 2
Alligator has undertaken discussions with the US Food and Drug Administration (FDA) and has been able to establish a clear development and approval pathway for mitazalimab in pancreatic cancer. Based on the emerging data from the OPTIMIZE-1 study, FDA has provided additional guidance and has endorsed OPTIMIZE-1 as a Phase 3 enabling study. Consequently, mitazalimab can proceed directly to a global Phase 3 registration study, which Alligator is preparing to initiate in early 2025.
1 Moreno, V., et al. Invest New Drugs 2023, 41, 93–104; https://doi.org/10.1007/s10637-022-01319-2.
2 Andersson, H., et al. Cells 2023, 12(19), 2365; https://doi.org/10.3390/cells12192365
3 Conroy, T., et al. N Engl J Med 2011, 364:1817-1825; https://doi.org/10.1056/nejmoa1011923
4 Wainberg, Z., et al. Lancet 2023, 402(10409):1272-1281; https://doi.org/10.1016/s0140-6736(23)01366-1